Tissue Processing, Embedding, and Sectioning
EAPL supports the research of Dr. Lambert (VR) and colleagues who focus on defining mechanisms through which human papilloma birus (HPV) influences development and progression of cancers of the anus, cervix, and oropharynx
EAPL processed and embedded tissues and generated serial sections from experimental and control mice
Supported by P01-CA022443 and P50-DE026787
Published in He et al. EMBO Mol Med, 2015 and den Boot et al. PNAS, 2015 with Newton (GEM) and Ahlquist (VR)

Immunohistochemistry (IHC) and Immunofluorescence (IF) Staining
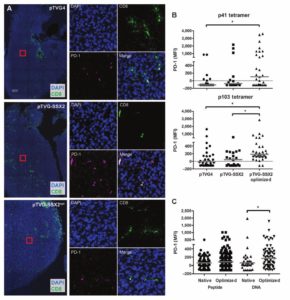
tissue samples from treated mice and controls. They also developed CD8 immunofluorescence assay (shown here).
EAPL supports the work of Dr. McNeel (DT) and colleagues who demonstrated that immunization with a DNA-based vaccine elicited PD-1 expression on antigen-specific CD8+ T cells
The EAPL developed a custom IHC protocol for CD8 and prepared histologic sections of tumors from treated mice and controls
These data directly contributed to the ongoing Phase I/II clinical trial in advanced prostate cancer of the UWCCC-developed DNA-based vaccine and the PD-1 inhibitor, pembrolizumab (NCT02499835)
Prostate Cancer Foundation Challenge Award
Published in Rekoske et al. Cancer Immunol Res, 2015
![]()
Special Stains
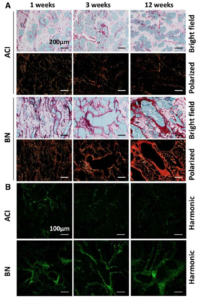
EAPL generated picrosirius red stained sections for Dr. Shull (GEM), Dr. Sullivan, and colleagues
This service enabled the use of second harmonic generation imaging microscopy to evaluate the extracellular matrix and associated collagens as a biomarker of susceptibility to estrogen-induced mammary cancer in genetically defined rat models
Supported by R01-CA077876; Susan G Komen Breast Cancer Foundation (KG081343); DoD Breast Cancer Research Program (W81XWH-11-1-0175)
Published in Ding et al. BMC Cancer, 2013 with Eliceiri (TM) and Sullivan.
More about EAPL
Prior Accomplishments
William Dove, PhD – Cancer Genetic & Epigenetic Mechanisms Program
Dr. Dove’s research team developed the Pirc rat as an alternative animal model of colon cancer. The Pirc rat harbors an ENU-induced mutation in Apc and develops macroadenomas and locally invasive adenocarcinomas of the colon at a high incidence (Figure 1; paraffin sections and H&E stained slides prepared by EPL). Importantly, the distribution of colon tumors in the intestinal tract of the Pirc rat closely resembles that of humans.
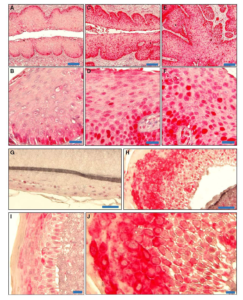
H&E stained slides (panels A-E) demonstrate invasion into the tumor stalk (A, B) and high grade dysplasia (C) in the Pirc rat tumors. Immunofluoresence staining for b-catenin reveals increased nuclear and cytoplasmic b-catenin in tumor cells in larger tumors (F, G) and in microadenomas (I, J). H&E stained tissues corresponding to the immunofluoresence images are shown in E and H.
Shannon Kenney, M.D. – Human Cancer Virology Program
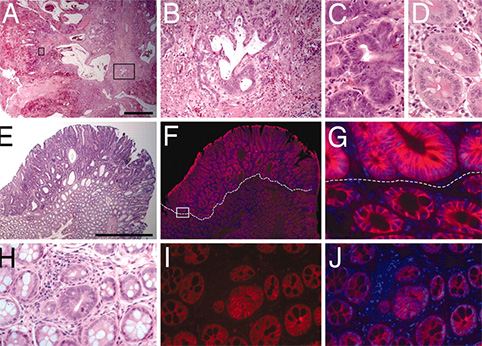
Dr. Kenney‘s team investigated the role of lytic viral infection in EBV pathogenesis and tumorigenesis in a new humanized NOD/LtSz- scid/IL2Rγnull (hNSG) mouse model that provides a more realistic immune background for EBV infection (Figure 2; paraffin sections prepared by EPL and H&E stained by EAPL). Infection appeared well controlled in some animals, but others eventually developed CD20+ diffuse large B cell lymphomas (DLBCL; tumors occurred more commonly in control vs. replication defective virus infected animals. Dr. Sullivan served as the consulting pathologist and co-author for this study.
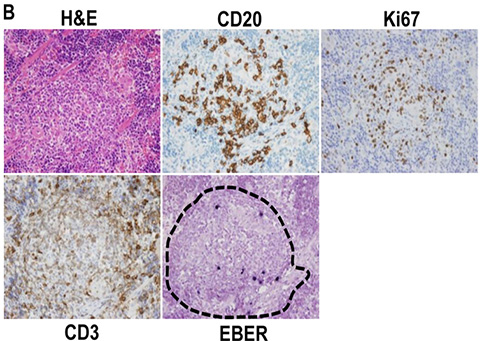
EBV-positive cells travel to specific regions of the reconstituted mouse spleen. H&E, EBV, CD20, CD3, and Ki67 staining was performed on spleen. EBV-positive cells were primarily located in the CD20-rich lymphoid zones (outlined with hatches) of the spleen.
![]()
Patricia Keely, PhD – Tumor Microenvironment Program
Advanced microscopy and analysis methods allow a wealth of molecular information to be obtained optically from tissues. Matthew Conklin, PhD of the Keely Laboratory has shown in paraffin sections prepared by the EAPL that neoplastic tissue in the MMTV-PyVT mouse model of mammary cancer differs from nearby normal mammary epithelium in the intensity and lifetime of NADH and FAD (Figure 3). This finding is consistent with the underlying metabolic differences that generally exist in neoplastic vs normal tissue and may provide an optical marker of neoplastic transformation.
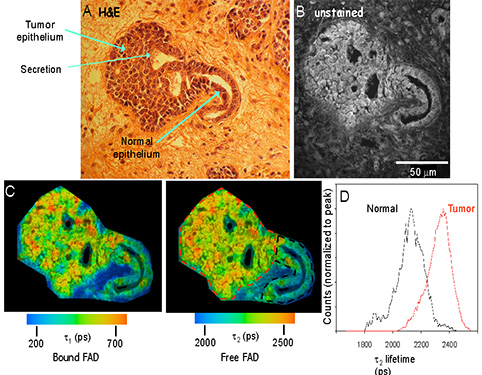
Fluorescence Lifetime Imaging Microscopy (FLIM) analysis of archived mouse samples: The fluorescent properties of normal and tumor epithelium differ. (A) H&E stained mouse mammary tumor showing normal and neoplastic tissues. (B) Fluorescence intensity image of the sequential unstained slide at 890nm excitation. (C) Color maps of the 1 (left) and 2 (right) components of the fluorescence lifetime, which illustrate the relatively longer lifetime values in tumor cells compared to normal epithelium. (D) Histogram analysis measuring the range of lifetime values of the two regions of interest drawn in (C) reveals the shift to longer lifetimes in tumor (red lines) cells compared to normal epithelium (color outline).